Decane Cracking Equation
пятница 12 октября admin 88
Cracking Of Nonane Equation. Cracking is conducted at high temperatures, by two processes. Steam cracking which produces high yields of alkenes; Catalytic cracking in which a catalyst is employed and which produces high yields of branched and cyclic. Cracking of decane. Chemical Equations. C 10 H 22-> 2C 5 H 11 (radicals) C 5 H 11. O lal meri pat rakhiyo by shazia khushk mp3 download mp3.
Word and Chemical Equations in Production of Materials Introduction This section will show all chemical equations including those that are not required for the HSC. A title will have the '(HSC)' flag if it is required for the HSC.
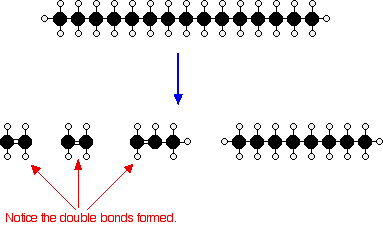
Cracking and alkenes is a reaction in which larger saturated are broken down into smaller, more useful hydrocarbon molecules, some of which are unsaturated: • the original starting hydrocarbons are • the products of cracking include alkanes and, members of a different For example, hexane can be cracked to form butane and ethene: hexane → butane + ethene C 6 H 14 → C 4 H 10 + C 2 H 4 The starting compound will always fit the rule for an alkane, C n H 2n+2. The first will also follow this rule. The second product will contain all the other C and H atoms.
Bela Bartok An Evening in the Village March 25, 1881 September 26, 1945) Hungariancomposer and pianist. One of the most important composers of the 20th century he andLisztare regarded as Hungary's greatest composers (Gillies 2001). Miscellaneous Romanian Folk Dances legal in U.S. Bela bartok romanian folk dances violin piano pdf but might not be in some European states.Romanian Folk Dances. We have another page with even more free Bartok sheet music. Bartok romanian folk dances violin piano sheet music. PDF scanned by Unknown Daphnis (2008/7/26) Pub lisher. Orchestration of the piano work Romanian Folk Dances, Sz.56: External Links Wikipedia article: Misc. Bela bartok romanian folk dances pdf to word. Romanian Folk Dances, No. 1—' By Bela Bartok Courtesy of Stick Game' The Sheet Music Archive Allegro moderato.
The second product is an alkene, so it will follow the rule C n H 2n. Question C 16 H 34 is an alkane which can be used as the starting chemical in cracking. One of the products of cracking this is an alkane which has 10 carbon atoms in it. Write a balanced symbol equation for this cracking reaction.
Reveal answer. Butane and ethene are produced Reasons for cracking Cracking is important for two main reasons: • It helps to match the supply of with the demand for them. • It produces alkenes, which are useful as for the industry. Supply and demand The supply is how much of a fraction an oil refinery produces. The demand is how much of a fraction customers want to buy. Very often, of produces more of the larger hydrocarbons than can be sold, and less of the smaller hydrocarbons than customers want.
Smaller hydrocarbons are more useful as than larger hydrocarbons. Since cracking converts larger hydrocarbons into smaller hydrocarbons, the supply of fuels is improved. This helps to match supply with demand. Alkenes Alkanes and alkenes both form homologous series of hydrocarbons, but: • alkanes are, their carbon atoms are only joined by C-C single bonds • alkenes are, they contain at least one C=C double bond As a result, alkenes are more than alkanes. Alkenes can take part in reactions that alkanes cannot. For example, ethene molecules can react together to form poly(ethene), a.
Alkenes will react with bromine water and turn it from orange/brown to colourless. This is the way to test for a double C=C bond in a molecule.